Quality and care safety policy
The Centre François Baclesse is committed to an overall strategy towards the improvement of care quality and safety.
The Quality / Risk Management strategy adopted at the Centre for over 15 years now guarantees users top-level care, guarantees professionals a high-performance working environment, and offers the institution a prime position in the regional healthcare system. The strategy is deployed by a vast number of professionals who are as active as they are committed.
Voluntary or mandatory certification has been accentuated (clinical research, biopathology department, tumour bank, data management centre) to now integrate patient care paths (European EUSOMA certification for the Normandy Breast Insitute), fundamental research and teaching (European accreditation as a Comprehensive Cancer Centre).
For the 2019-2022 period, the Centre François Baclesse has identified the following priorities for improvement in its hospital establishment project:
- Reformed coordination by integrating all certification and accreditation processes;
- Deployment of improvement opportunities in response to HAS (French National Authority for Health) certification and IQSS (care quality and safety indicators)
- Support for the ambitions outlined in the medical and scientific project, at the heart of the centre’s strategy;
- Optimisation of quality and risk management tools and organisation;
- Continuation of cultural integration and empowerment of all involved persons;
- Increased collaboration with users via reinforced integration of strategic processes in the field of care quality and safety;
- Measurement of results of delivered quality.
Our quality strategy
The Centre François Baclesse places quality among its 4 key values and materialises this ambition through its commitment to a number of quality-related action plans:
- European Comprehensive Cancer Centre accreditation for all the centre’s activities (care/teaching/research) by the OECI (Organisation of European Cancer Institutes),
- HAS (French National Authority for Health) certification level A obtained in November 2019 as per the V2014 reference document,
- European EUSOMA certification for the INS (Normandy Breast Institute), guaranteeing quality care for breast cancer,
- NF EN ISO 15189 (COFRAC) accreditation for the biology/cancer genetics laboratory and the anatomopathology department,
- ISO 9001 certification for clinical research, including the management and dispensing of experimental medicines,
- ISO 9001 for the data processing centre,
- NFS 96-900 certification for the tumour bank and the OVA Ressources biological resource centre,
- QUALIOPI for the centre’s continuing education activity,
- CLIP2 early trial centre certification by the INCa.
Many staff members are involved in coordinating this quality strategy, and each sector of activity has integrated its own continuous improvement programme with regard to professional practices and care safety.
Every year, the centre develops an ambitious quality training plan. The Quali-Day one-day event, specifically devoted to developing quality culture, reunites over 500 professionals over a programme of workshops and conferences.
The level achieved by our establishment is objectified through the results of care quality and safety indicators that can be consulted on line via the Scope Santé website: www.scopesante.fr
Patients and users of the centre are also involved in developing care quality and safety:
- by reporting any shortcomings they may observe, to ensure they are dealt with and corrected by the establishment’s quality department,
- in partnership with the patient satisfaction assessment initiative (e-Satis),
- by enabling reliable identification at all stages in care, via the provision of a formal identity document with photograph upon creation of the medical file,
- by informing healthcare professionals of any ongoing medical treatment or any event likely to influence care.
User representatives who attend the users’ committee meetings play an active role in the development and deployment of the policy towards improved and safe patient care. They are prime contacts in relations between patients and users with the institution.
If you would like to tell us about your own experience, suggest avenues for improvement in global patient care, please feel free to CONTACT US!
Certification and accreditation
The Centre François Baclesse boasts national and international renown for its quality care and research, thanks to its extensive certification and accreditation.

Comprehensive Cancer Centre accreditation – Centre François Baclesse
26 June 2020 – The Centre François Baclesse was accredited as a ‘Comprehensive Cancer Centre’ by the Organisation of European Cancer Institutes (OECI), hence becoming the 5th French cancer centre to obtain this label, synonymous to excellence.
This recognition of quality is reserved for multidisciplinary cancer institutions which integrate cancer care, research and training. Only those which can justify a vast range of medical and technical skills, including clinical and translational research, are likely to obtain certification as a Comprehensive Cancer Centre. The excellence and the quality of global patient care at the Centre François Baclesse, at all phases of the care path, together with access to innovation, are praised via this certification.
This European accreditation, valid for 5 years, is the result of a policy towards continuous improvement integrated within the Centre François Baclesse hospital establishment project. It offers the centre international exposure, propitious for developing new research projects and new interactions to benefit patients.

EUSOMA accreditation for the Centre François Baclesse Normandy Breast Institute
22 April 2020 – The European Society of Breast Cancer Specialists (EUSOMA) awarded the Normandy Breast Institute (INS – Institut Normand du Sein) at the Centre François Baclesse with its European certification.
This certification, the most demanding at European level, materialises recognition of care and management of breast cancers within the institute. It requires, not only for all the necessary diagnostic, technical and treatment techniques, but also a database to enable the collection and prospective evaluation of the necessary indicators to monitor care quality.
The 1st French centre to obtain EUSOMA certification
Every year, the Normandy Breast Insitute, located within the Centre François Baclesse, treats around 1,000 women presenting with breast cancer at regional level, for whom it ensures long-term follow-up. This experience, and the will to provide top-quality care through global patient management (via, not only, all available medico-surgical techniques, but also supportive care) enabled the INS to become the very first French centre to obtain such certification.
HAS V2014 Certification – Centre François Baclesse
23 October 2019 – The Centre François Baclesse was awarded level A certification – the highest level – from the French National Authority for Health (HAS), valid for 6 years.
The HAS certification is an independent procedure of mandatory evaluation of the level of quality and safety of care dispensed in French public and private healthcare establishments. It is conducted by professional peers (expert visitors) who are commissioned by the HAS.
This certification focuses particularly on the evaluation of the establishment’s capacity to identify and control its risks and to implement good practice.
This excellent result is the product of an investment by the entire staff at the centre. It materialises all efforts and actions aimed at improving the safest global patient care.
The centre’s certification report can be consulted on the HAS website:

NF EN ISO 15189 accreditation – Cancer biology and genetics laboratory and anatomopathology department at the Centre François Baclesse (Department of Biopathology)
Since 1 July 2014, the Cancer Genetics and Biology Laboratory (LBGC – Laboratoire de Biologie et de Génétique du Cancer) is accredited by the COFRAC.
The deployment of the quality policy within the anatomopathology/cytopathology laboratory enabled the accreditation of the Biopathology department under no. 8-3264. In July 2024, the Biology laboratory obtained NF EN ISO 22870 v2017 accreditation for off-site biology.
Scopes are available on the Cofrac websitehttps://www.cofrac.fr/.

ISO 9001 certification – Clinical Research and the clinical trials sector of the hospital Pharmacy
In 2017, the French Standardisation Association (AFNOR) awarded ISO 9001: 2015 certification to the Centre François Baclesse Clinical Research Unit and the clinical trials sector of the hospital Pharmacy department. The same certification was renewed in October 2020.
The attribution of this international certification offers acknowledgment of the quality of our clinical research activity, equally for patients, physicians and academic or industrial partners.
Such recognition offers proof of the Centre François Baclesse’s commitment to a programme of continuous quality improvement, implemented through a process-based and risk management strategy.

ISO 9001 Certification – Data management centre
14 May 2018 – The Cancéropôle Nord-Ouest (CNO) Data Management Centre (CTD – Centre de Traitement des Données) is awarded ISO 9001 certification (obtained in March 2018).
Located within the François Baclesse Comprehensive Cancer Centre, the CTD-CNO was awarded ISO 9001 (2015 version) certification by Bureau Veritas Certification for its activities in the administration and management of research project data. This internationally renowned standard guarantees for all – patients, physicians and academic partners, a high level of quality and safety in the organisation and processing of clinical trial data.
Through such deployment, the CDT has endowed itself with tools aimed at improving provided services and at adapting to suit the needs and expectations of its partners and funders, all through a context of permanent regulatory change.

NFS 96-900 certification for the Tumour Bank and the OvaRessources Biological Resource Centre
The Caen Lower Normandy Tumour Bank (a structure shared with the CHU university hospital) and the OvaRessources Biological Resource Centre (on ovarian cancers) are certified together, according to the NF S 96-900 standard, since 2016.
This certification offers recognition of the quality of our activity in the preparation, conservation and provision of biological resources for research purposes (and for care for the TCBN tumour bank) to benefit patients, researchers and our public or private partners.
Awarded by the AFNOR, it enables risks to be controlled thanks to reliable processes, together with traceability and sample quality for all professionals involved in research.

Care quality and safety indicators
The Centre François Baclesse participates in the collection of national indicators developed by the HAS and aimed at the improvement of care management quality and the prevention of nosocomial infections.
Indicator results
Consult the care quality and safety indicator results for the François Baclesse Centre on the national QualiScope website: View form.
Processing of personal data within the framework of indicator collection
Within the context of care quality and safety indicator collection, the HAS may be brought to collect and use indirectly nominative personal data on patients, drawn randomly by healthcare establishments. The HAS is responsible for the processing of any such data.
For further information: Information note for hospitalised patients whose medical file is likely to be subject to data processing, within the context of the collection of care quality and safety indicators. View note.
Hospitalised patient satisfaction
In order to improve the quality of service and care offered to patients, our establishment is actively involved in the national e-Satis programme to measure the satisfaction of hospitalised patients.
Within the framework of this survey, you will be invited by mail to give your own view on the quality of your overall care, via a totally unanimous on-line questionnaire.
Results of the national patient satisfaction survey: e-Satis 2021
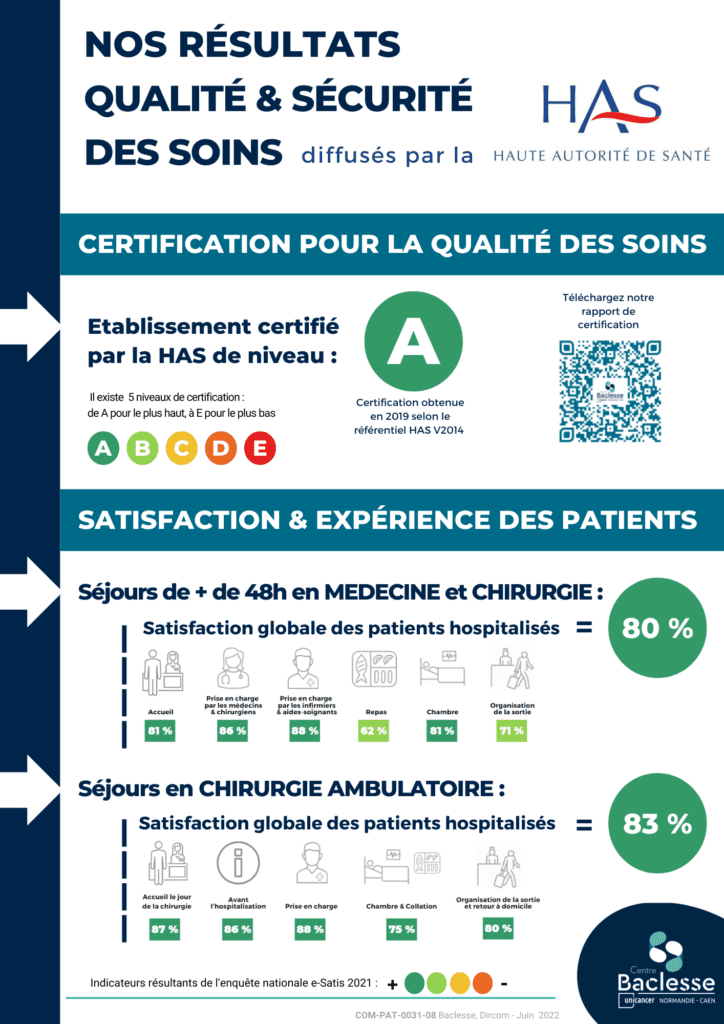
All results for the Centre François Baclesse and for other healthcare establishments can be consulted at QualiScope.
Quali’Day: a day devoted to care quality and safety
The centre’s quality policy is brought to life through the yearly organisation of a one-day training event for its staff devoted to care quality and safety. This institutional event, referred to as Quali’Day, was created in 2011.
The aim of Quali’Day is to federate professionals from the Centre François Baclesse, to exchange and share on care-related themes and on action engaged to improve overall patient management.
A total of 650 people take part in this one-day event, during which conferences and workshops are organised.
Care safety
Care-related adverse events (CRAE)
Care-related adverse events (CREAs) cover any failings / shortcomings / sometimes errors that negatively impact patient care paths, the majority of which can be avoided. Be they in the form of failings in patient management, identity-related errors, medicinal drug-related accidents, etc. they are defined as adverse events for the patient, with a certain degree of seriousness and associated with care provided during investigations, treatment or prevention procedures.
Notification of care-related adverse events by any healthcare professional or user enables patient safety to be constantly improved, through the lessons learned from their analysis and the implementation of action towards improvement.
The centre’s patients and users who notify our professionals of any shortcomings they may encounter play an active role in improving care quality and safety.

You can notify the Centre François Baclesse by mail of any care-related adverse event, in order for it to be dealt with by the establishment’s Quality and Risk Management department.
Pharmacovigilance: notification of adverse effects associated with medicinal treatment
The aim of pharmacovigilance is to monitor medicinal drugs and to prevent the risk of adverse effects resulting from their use, whether the risk is potential or confirmed. This provides a guarantee that remains valid throughout the life cycle of the drug in question.
Whenever there is a suspected link between an adverse effect and medication, even if not confirmed, it must be declared to the regional pharmacovigilance centre (CRPV). Declarations forwarded to the CRPV are then recorded by the ANSM – the French national agency for drug and health product safety – which ensures national and collective monitoring of health products, analyses notifications and, if required, takes the necessary measures to reinforce patient safety. The declaration of adverse effects is an essential step towards progress in patient safety, for it enables industrial stakeholders to improve the use of their products.

As a patient, you can – in a few clicks – notify the healthcare authorities of any adverse event associated with the use of a medicinal drug or any other health product, by completing the notification form on the following website: signalement.social-sante.gouv.fr
It is recommended that you notify your treating physician of any adverse events at the same time.
Prevention of healthcare-related infections
Healthcare-related infections (HCRIs) are infections acquired during healthcare. HCRIs acquired in hospital are called nosocomial infections. These infections can be caused by your own germs (naturally present on your skin, in your digestive tube, your mouth, etc.) or by germs transmitted from the hands of caregivers, medical material or the environment.
Their transmission can be facilitated during complex care procedures and by a fragile state of health. Preventive measures exist to avoid them.
How we prevent them
Prevention by caregivers
Preventive measures must be applied by all caregivers for all care procedures on all patients, independently of the site where they are conducted (including home care): they are referred to as standard precautions.
The principle of these precautions is the disinfection of hands, without jewellery, with a sanitising solution. Other measures concern abidance by aseptic rules during care, the disinfection of material and the cleaning of premises.
Prevention by patients
You can also help us to lower the risk of care-related infection and avoid the dissemination of germs in the environment by abiding by a few simple hygiene rules:
- hand hygiene: sanitising gel is also there for you to use – before and after meals, after going to the toilet or wiping your nose, when you leave and when you return to your room (if your hands are dirty, please wash them with soap and water),
- couging or sneezing: cover your mouth with your hand or a single-use handkerchief, throw soiled handkerchiefs in the waste bin, wear a surgical mask outside your room if you are coughing a lot (ask the care team),
- touching venous or urinary catheters, dressings, etc. increases the risk of contamination. If you are feeling uncomfortable with any of these devices, please inform the care team and the necessary measures will be taken,
- special instructions may be given to you depending on the reason for your hospitalisation or your state of health: for your health and safety, please follow them.
Prevention by visitors
In order to avoid bringing germs into the hospital or taking them home, visitors must also apply precaution: for your health and theirs, ask them to abide by them!
- ask your friends and family to postpone their visit if they are ill (cold, gastroenteritis, etc.) or in the company of fragile or sick children. If the visit cannot be postponed, please inform the care team and ask what measures must be taken (medical mask, etc.),
- ask them to rub their hands with sanitising gel, when they enter your room.
- generally speaking, it is preferable to limit the number of visitors in rooms (the more people, the more germs…),
- the soil in potted plants contains germs that can be dangerous for your health: please ask your visitors to bring an alternative (cut flowers, confectionery, etc.),
- inside your room:
- to guarantee quality and safety in all care dispensed to you, visitors must leave your room whenever such care is administered and only the care team is authorised to touch medical equipment,
- to avoid contamination, please ask your visitors to use the public toilets rather than those in your room, to sit on chairs rather than on the bed and to follow any instructions given by the care team,
- when they leave your room, they can rub their hands again with the sanitising gel.
What about organisation
A Nosocomial Infection Control Committee (NICC), comprised of representatives from the management department, medical and paramedical staff members and user representatives, devises a pluri-annual action plan including, in particular, the following priorities:
- professional training,
- assessment of daily practice,
- drafting of procedures for dispensing care,
- microbiological monitoring of the environment,
The chosen themes for such action change in line with national strategies and needs identified within the establishment. In particular, they cover the following points:
- to develop the prevention of infection associated with long care paths, by involving patients,
- reinforcing prevention and management of bacterial resistance to antibiotics.
- reducing the risk of infection associated with invasive procedures (surgery, venous implant, endoscopy, etc.).
In order to implement its action plan, the NICC relies on the Operational Hygiene Team, comprised of healthcare professionals qualified in the prevention of infectious risk.
Patient identity vigilance
For all professionals, your safety is a fundamental priority throughout your care at the Centre François Baclesse. The centre has implemented organisation which enables you to be reliably identified at each phase of your care path: this is what we refer to as patient identity vigilance. At the Centre François Baclesse, the Patient Identity Vigilance Cell is in charge of implementing a system to monitor and prevent errors and risks associated with patient identification.
Your identification is the first essential and fundamental step before any care procedure. This is why:
- a formal identity document is requested upon creation of your file,
- an identification band is placed on your wrist for all hospitalisations,
- your surname, first name and birth date will frequently be requested at each step in your care path.
Please feel free to confirm your identity at each new step in your care path.

Transfusion safety and haemovigilance
The Haemovigilance Unit at the Centre François Baclesse enables optimised safety in the transfusion of the labile blood products you may need. Thanks to strict and regulated medical control, from prescription to administration of the blood product and during post-transfusion monitoring, the organistion of this unit at the centre is perfectly in abidance with safety standards pertaining to blood transfusion.

