The new clinical research care unit
Since 2 May 2022, a new care unit has been welcoming clinical research patients on the Centre’s 2nd floor. It is the ‘Unité d’Investigation, de Recherche, d’Innovation et de Soins – IRIS’ (Investigation, Research, Innovation and Care Unit).
Patients included in clinical trials are now treated specifically in this unit. The IRIS Unit benefits from:
- its own sampling centre,
- medical and paramedical consultation rooms,
- 10 day hospital beds/places,
- and a team of specific healthcare professionals, experts in clinical research.
How is patient care organised in the IRIS Unit?
First and foremost, the medical oncologist needs to present and explain the trial to the patient, who, if willing to participate, signs formal consent. The patient is then included in the study. Then:
- The patient is welcomed by the nursing team at the sampling centre. Prior to any medical consultation, vital signs are checked (pulse, blood pressure, temperature), and tests are conducted (electrocardiogram – ECG for example).
- The administration of treatment is conducted under strict surveillance (several times per hour) by the nursing and medical team (measurement of vital signs, how the patient is feeling, blood tests, etc.).
Monitoring of patients included in clinical trials can last several years, in order to assess the efficacy of treatments and to measure their potential mid- or long-term toxicity. All clinical research activities around patient care are conducted within this unique unit, specifically devoted to clinical research.
The advantages of the IRIS Unit
This new department, organised within a propitious environment for safe patient care, enables:
- the improvement of patient comfort and of staff working conditions,
- more fluid care paths for clinical research patients.
‘I am delighted to see this IRIS Unit open, and to enable access to the most innovative cancer care to be optimised, with the best possible comfort for our patients,’ Dr Mélanie Dos Santos, head of the IRIS care unit, explains.
Inauguration of the IRIS Unit on 13 May 2022 The unit was inaugurated on Friday 13th May, in the presence of members of the Centre’s Board, the press and invited personalities. The inauguration began in the board room with speeches by Dr Carine Segura-Djezzar, President of the Medical Committee, Prof. Florence Joly, head of the Education, Research and Innovation department, and Prof. Mahé, CEO.
Clinical research organisation at the François Baclesse Comprehensive Cancer Centre
The François Baclesse Centre’s clinical research department is placed under the responsibility of Prof. Florence Joly, head of the Education, Research and Innovation department. It is comprised of:
- an investigation unit (IRIS Unit)
- a promotion unit
The IRIS Unit
Care for patients included in type 1 interventional research studies on medicinal treatments or innovative therapies is ensured by a specific medical and paramedical team.
This organisation model enables the care of patients participating in research, from Monday to Friday, 8.30am to 7pm. Outside of the aforementioned hours, and on weekends or public holidays, continuous care is provided by the medical and paramedical teams in the care units initially allocated for the given patient, by the on-call intern and by an on-call senior physician, as per the establishment’s procedures.
Context around the creation of the IRIS Unit
For over twenty years, the François Baclesse Comprehensive Cancer Centre has been a major stakeholder in clinical research in the field of oncology.
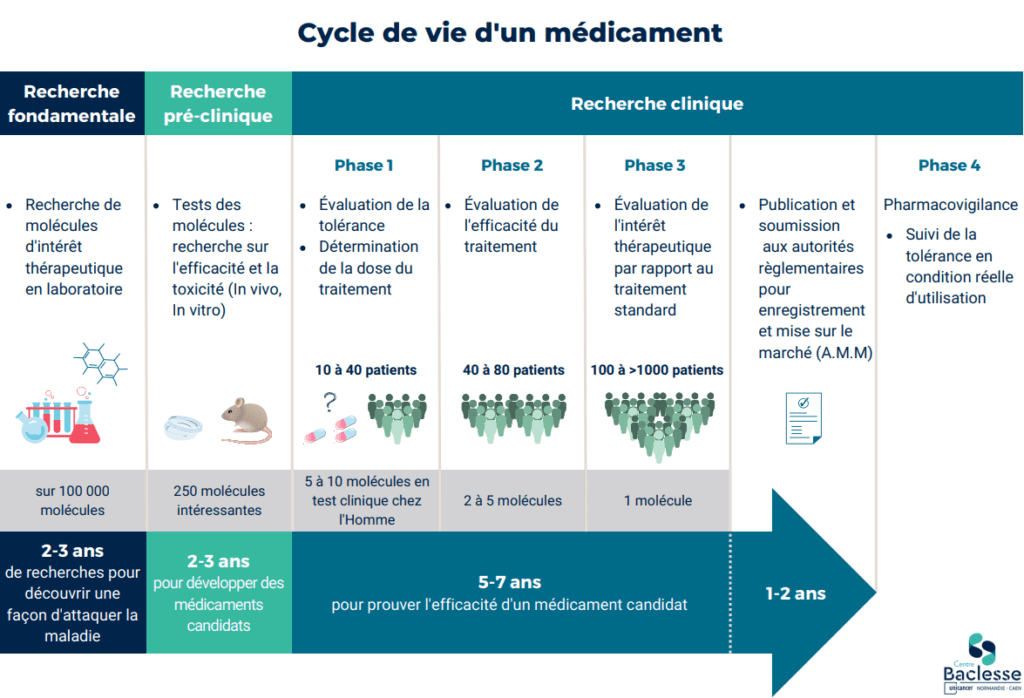
The Centre’s clinical research department takes on all types of interventional studies, from phase I to phase IV, placing priority on early phase studies. Annually, 170 intervention trials are open to inclusions, hence enabling regional patients to be offered access to novel treatments in a large number of therapeutic indications.
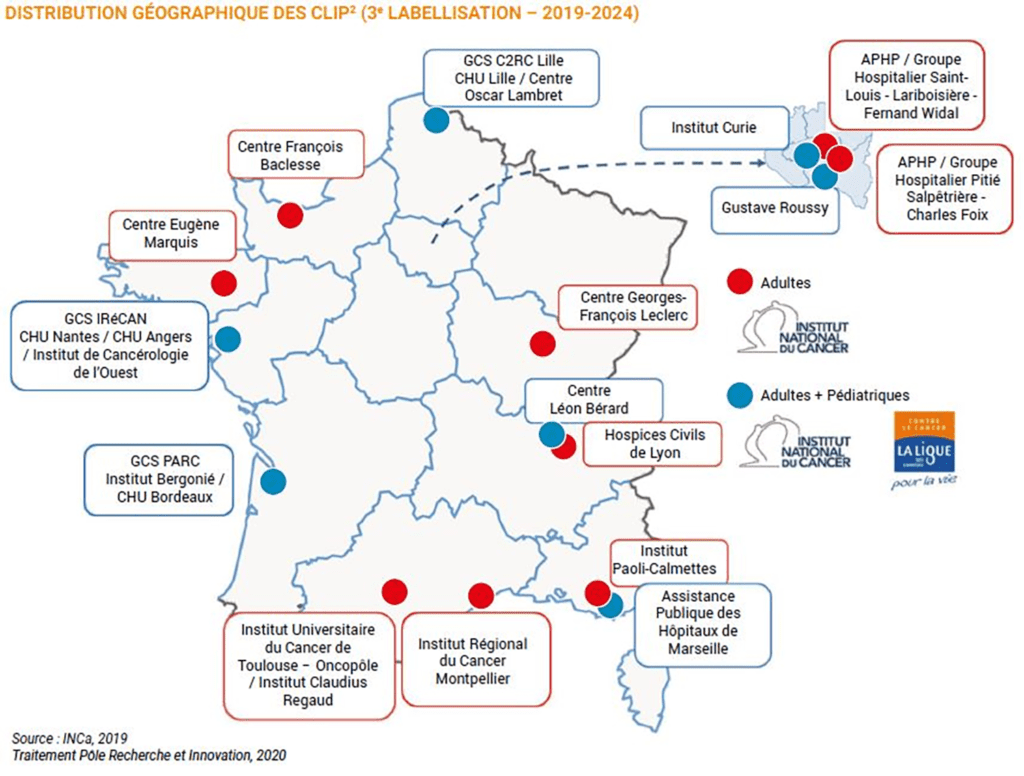
In 2019, for the third time running since 2010, the François Baclesse Centre was awarded the CLIP² label (INCa-designated early-phase trial centre) in adult oncology, by the INCa – the French National Cancer Institute. Baclesse is the only certified centre in Normandy. This label distinguishes the 16 best centres in France with the capacity to conduct early phase clinical trials in oncology, hence offering their patients access to therapeutic innovation. The aim of early phase trials is to evaluate the safe use of a new drug (administered alone or associated with other treatments) and to gather data on its anti-tumour activity. These trials are essentially proposed to patients for whom they offer an interesting healthcare alternative, or after conventional treatment failure. The certification procedure has already enabled the inclusion of over 400 patients in more than 80 early phase trials over a period of 4 years.
Since 2017, clinical research at the François Baclesse Centre is also certified ISO 9001:2015 by AFNOR, the French Standardisation Association. This internationally acknowledged standard guarantees for all – patients, physicians, academic and industrial partners alike – a high level of quality and safety in the organisation of clinical trials.
At the François Baclesse Centre, care for patients participating in interventional research on healthcare products is ensured by a specific medical and paramedical team with support from the Centre’s other staff. This activity was previously integrated within the day hospital, located on the ground floor and comprising a surface area of 80m2, with 6 specifically designated beds.
As from 2 May 2022, the entire clinical research team will be reunited on the establishment’s 2nd floor, and will benefit from an area of 310m2, equipped with 10 in-patient beds, hence enabling capacity to be increased in line with the activity’s evolution and organisation of patient care and monitoring to be improved.
‘It is the materialisation of an approach initiated by the establishment over 10 years ago, aimed at integrating therapeutic innovation in patient care. This new department will enable us to pursue our ambitions by offering more patients access to clinical research,’ notes Professor Florence Joly, head of the Centre François Baclesse Education, Research and Innovation department.
Why develop clinical research at Baclesse?
Research is one of the three missions entrusted to France’s CLCCs (cancer care centres), together with patient care and education.
Patient participation in clinical trials is an essential contribution towards the development of new treatments and new therapeutic strategies. These innovative therapies can benefit a great number of people affected by cancer. Early phase trials offer patients access to innovative treatment that is not yet available on the market. Advances in treatment for cancer are the result of clinical research, thanks to rigorously and methodically conducted trials, hence guaranteeing an excellent level of scientific proof. Today’s treatments are yesterday’s clinical trials.
The aims of clinical research:
- To advance medical knowledge
- To offer patients access to innovative treatments well before they are marketed (more efficient, better tolerated)
- To determine the best therapeutic strategy (to establish systems of reference)
Within the context of therapeutic trials, treatments can be:
- New molecules,
- Drug associations,
- New ways to administer drugs or new treatment techniques (surgery, radiotherapy, etc.).